How Many Neutrons Does Oxygen Have in Its Nucleus
Oxygen is different from carbon because it has more particles. Oxygen-16 16O is a stable isotope of oxygen having 8 neutrons and 8 protons in its nucleus.

Pin On Atomic Structure Protons Neutrons And Electrons
That should answer your question.

. An Na ion is a sodium atom that has lost one electron as that makes the number of electrons in the atom equal to that of the nearest Nobel gas Neon which has 10 electrons. On the other hand Na has 11 protons 10 electrons and 12 neutrons. Light oxygen-16 with 8 protons and 8 neutrons is the most common isotope found in nature followed by much lesser amounts of heavy oxygen-18 with 8 protons and 10 neutrons.
It has a mass of 1599491461956 u. Atomic mass is simply the sum of the protons and neutrons in the nucleus -- here 17 total. The atomic mass of oxygen is 16.
About one out of every 1000 oxygen atoms contains 2 additional neutrons and is. How many neutrons does oxygen have in its nucleus. Oxygen is a stable isotope of oxygen with a nucleus of 8 neutrons and 8 protons.
How Many Protons Does Carbon Have. All oxygen atoms have 8 protons but the nucleus might contain 8 9 or 10 neutrons. A is the number of neutrons plus protons in the nucleus.
Light oxygen-16 with 8 protons and 8 neutrons is the most common isotope found in nature followed by much lesser amounts of heavy oxygen-18 with 8 protons and 10 neutrons. In each carbon atom there are 6 protons 6 electrons and 6 neutronsApr 9 2019. Consider fluorine atoms with 9 protons and 10 neutrons.
All oxygen atoms have 8 protons but the nucleus might contain 8 9 or 10 neutrons. March 24 2022. A single oxygen atom has eight protons eight electrons and eight neutrons.
So there are 178 or 9 neutrons in the nucleus of oxygen 17. The frequency of occurrence of these isotopes varies somewhat depending on sample. The importance of protons.
A nitrogen atom has 7 protons and its most common isotope has 7 neutrons. The answer is C. Why nitrogen has 7 atomic number.
Protons are the smallest unit of an atom and are responsible for the nucleus of an atom. In the nucleus of an oxygen-18 atom there are 10 neutrons while in the nucleus of an oxygen-16 atom there are only 8 neutrons. This particular isotope of oxygen has an atomic mass of 17.
Light oxygen-16 with 8 protons and 8 neutrons is the most common isotope found in nature followed by much lesser amounts of heavy oxygen-18 with 8 protons and 10 neutrons. Oxygen atomic symbol O has atomic number eight in the periodic table. March 8 2022.
Its mass is 1599491461956 u. Oxygen-16 16O is a stable isotope of oxygen having 8 neutrons and 8 protons in its nucleus. Nitrogen has an atomic number of 7 Z7 because it has 7 protons in its nucleus.
We got the mass as 16 units and so itll have 1688 neutrons. Oxygen has an atomic number of 8 and the mass number of an atom is the sum of its atomic number plus its neutron number. An oxygen atom has 8 positive protons and 8 neutrons in its nucleus and 8 negative electrons speeding around the nucleus.
All oxygen atoms have 8 protons but the nucleus might contain 8 9 or 10 neutrons. Light oxygen-16 with 8 protons and 8 neutrons is the most common isotope found in nature followed by much lesser amounts of heavy oxygen-18 with 8 protons and 10 neutrons. Oxygen has eight protons eight neutrons and eight electrons.
Dear Student CO2 is composed of three atoms one carbon and two oxygen. March 22 2022 By. Oxygen by definition has 8 protons in its nucleus.
In each carbon atom there are 6 protons 6 electrons and 6 neutrons. A radioactive isotope of nitrogen has 9 neutrons. Stable oxygen atoms may contain 8 9 or 10 neutronsby far over 997 the most common is with 8 neutrons next about 02 is 10 neutrons.
Dear Student CO 2 is composed of three atoms one carbon and two oxygen. All oxygen atoms have 8 protons. Some nitrogen atoms have an atomic mass number of 15 A15.
And we know that the atomic number of nitrogen is 7 so the number of protons in nitrogen is 7The number of electrons in an atom is always equal to the number of protons. The sodium atom has 11 protons 11 electrons and 12 neutrons. Most of the oxygen in water molecules is composed of 8 protons and 8 neutrons in its nucleus giving it a mass number the number of protons and neutrons in an element or isotope of 16.
All oxygen atoms have 8 protons but the nucleus might contain 8 9 or 10 neutrons.

Protons Neutrons Electrons Modelos Atomicos Atomo Ciencias Naturais

Atomic Structure Of Carbon Atomic Structure Carbon Atom Model Atom Model

Atoms Lesson Plan A Complete Science Lesson Using The 5e Method Of Instruction Kesler Science Science Lessons Science Lesson Plans Kesler Science

How Atoms Work Bohr Model Atom Model Project Atom Model
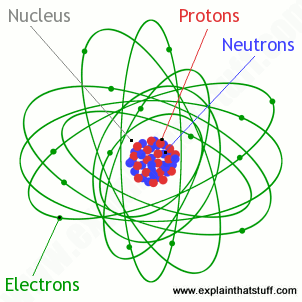
Atoms What Are They What S Inside Them Explain That Stuff Proton Neutron Electron Atom Montessori Elementary

Basic Chemistry Atoms And Ions Chemistry Basics Chemistry Education Chemistry Lessons

All Oxygen Atoms Have 8 Protons But The Nucleus Might Contain 8 9 Or 10 Neutrons Light Oxygen 16 With 8 Protons And 8 Neutron Neutrons 10 Things Protons

Protons Neutrons Electrons And Isotopes Youtube Neutrons Protons Electrons

Diagram Of An Oxygen Atom Atom Project Atom Model Project School Science Projects

Mcdonald Publishing Atoms Elements Molecules Compounds Poster Set Atoms And Molecules For Kids Chemistry Education Teaching Chemistry

Oxygen Was Discovered In 1774 By Joseph Priestley In England And Two Years Earlier But Unpublished By Carl W Scheele In Sweden Atom Model Oxygen Atom

Nitrogen Atomic Structure Atomic Structure Nitrogen Protons

Calculating Number Of Protons Neutrons And Electrons Google Search Relative Atomic Mass Neutrons Protons

Oxygen Shells Chemistry Projects Oxygen Binding Energy

Oxygen Shells Oxygen Chemistry Projects Binding Energy

Atomic Structure Of Oxygen Atomic Structure Atom Atom Model

Model Of An Oxygen Atom 8 Neutrons 8 Protons And 8 Electrons All Wooden Attached On A Candle Holder Science Projects Science For Kids Fun Science

Products Detail Variquest Com Structure Of Matter Teaching Chemistry Visual Learning Tools

Comments
Post a Comment